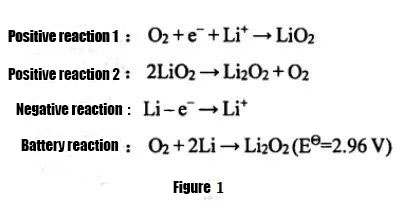01 Menene batirin lithium-air da batirin lithium-sulfur?
① Li-air baturi
Batirin lithium-iska yana amfani da iskar oxygen a matsayin ingantacciyar amsawar lantarki da kuma ƙarfe lithium azaman wutar lantarki mara kyau.Yana da babban ka'idar makamashi yawa (3500wh/kg), kuma ainihin ƙarfin ƙarfinsa zai iya kaiwa 500-1000wh/kg, wanda ya fi tsarin baturi na lithium-ion na al'ada.Batirin lithium-air sun ƙunshi ingantattun na'urorin lantarki, masu amfani da lantarki da na'urorin lantarki marasa kyau.A cikin tsarin baturi marasa ruwa, a halin yanzu ana amfani da oxygen mai tsabta azaman iskar gas, don haka ana iya kiran batir lithium-air baturi na lithium-oxygen.
A cikin 1996, Abraham et al.yayi nasarar harhada batir lithium-air na farko mara ruwa a cikin dakin gwaje-gwaje.Daga nan sai masu bincike suka fara mai da hankali kan halayen lantarki na ciki da tsarin batir lithium-air mara ruwa;a 2002, Read et al.gano cewa aikin electrochemical na batirin lithium-air ya dogara ne akan kaushi na electrolyte da kayan cathode na iska;in 2006, Ogasawara et al.amfani da Mass spectrometer, a karon farko an tabbatar da cewa Li2O2 ya kasance oxidized da iskar oxygen da aka saki a lokacin caji, wanda ya tabbatar da electrochemical reversibility na Li2O2.Don haka, batir lithium-air sun sami kulawa mai yawa da haɓaka cikin sauri.
② baturin lithium-sulfur
Batirin Lithium-sulfur shine tsarin baturi na biyu dangane da jujjuyar amsawar takamaiman ƙarfin sulfur (1675mAh/g) da ƙarfe lithium (3860mAh/g), tare da matsakaicin fitarwar wutar lantarki na kusan 2.15V.Its theoretical yawa makamashi iya isa 2600wh/kg.Kayan albarkatunsa yana da fa'ida na ƙarancin farashi da kuma abokantaka na muhalli, don haka yana da babban damar ci gaba.Ƙirƙirar batirin lithium-sulfur za a iya gano shi tun a shekarun 1960, lokacin da Herbert da Ulam suka nemi takardar izinin batir.Samfurin wannan baturi na lithium-sulfur yayi amfani da lithium ko alloy lithium a matsayin abu mara kyau, sulfur a matsayin tabbataccen abu na lantarki kuma ya ƙunshi aliphatic saturated amines.na electrolyte.Bayan 'yan shekaru, an inganta batura lithium-sulfur ta hanyar gabatar da kaushi mai ƙarfi kamar PC, DMSO, da DMF, kuma an sami batir 2.35-2.5V.A ƙarshen 1980s, an tabbatar da ethers suna da amfani a batura lithium-sulfur.A cikin binciken da ya biyo baya, gano tushen ether, yin amfani da LiNO3 a matsayin ƙari na electrolyte, da kuma shawarar carbon/sulfur composite tabbatacce electrodes sun buɗe haɓakar bincike na batir lithium-sulfur.
02 Ka'idar aiki na batirin lithium-air da baturin lithium-sulfur
① Li-air baturi
Dangane da jihohi daban-daban na electrolyte da aka yi amfani da su, ana iya raba batirin lithium-air zuwa tsarin ruwa, tsarin halitta, tsarin samar da ruwa-kwayoyin halitta, da batir lithium-air mai ƙarfi.Daga cikin su, saboda ƙarancin ƙayyadaddun ƙarfin batirin lithium-air ta amfani da electrolytes na tushen ruwa, matsalolin kariya ga ƙarfe na lithium, da rashin jujjuyawar tsarin, batirin lithium-air ɗin da ba na ruwa ba da duk wani ƙarfi-jihar lithium-air. an fi amfani da batura a yanzu.Bincike.Abraham da Z.Jiang ne suka fara samar da batir lithium-air marasa ruwa a cikin 1996. An nuna ma'aunin amsawar fitarwa a cikin hoto na 1. Halin cajin shine akasin haka.Electrolyte yafi amfani da Organic electrolyte ko m electrolyte, kuma fitarwa samfurin yafi Li2O2 , samfurin ne insoluble a cikin electrolyte, kuma yana da sauki tara a kan iska tabbatacce lantarki, rinjayar da fitarwa iya aiki na lithium-air baturi.
Batirin lithium-air yana da fa'ida ta ultra-high makamashi yawa, abokantakar muhalli, da ƙarancin farashi, amma har yanzu binciken su yana kan ƙuruciya, kuma har yanzu akwai matsaloli da yawa da za a warware su, kamar catalysis na rage iskar oxygen, iskar oxygen permeability da hydrophobicity na iska electrodes, da deactivation na iska lantarki da dai sauransu.
② baturin lithium-sulfur
Lithium-sulfur baturi yawanci amfani da elemental sulfur ko sulfur tushen mahadi a matsayin tabbatacce electrode abu na baturi, kuma karfe lithium yawanci amfani da korau electrode.A lokacin aikin fitarwa, ƙarfen lithium ɗin da ke cikin gurɓataccen lantarki yana oxidized don rasa electron kuma ya haifar da ions lithium;Daga nan sai a tura electrons zuwa na'urar lantarki ta hanyar da'ira ta waje, sannan kuma ions lithium da aka samar suma ana tura su zuwa ma'auni mai kyau ta hanyar electrolyte don amsawa da sulfur don samar da polysulfide.Lithium (LiPSs), sa'an nan kuma ƙara mayar da martani don samar da lithium sulfide don kammala aikin fitarwa.A yayin aiwatar da caji, lithium ions a cikin LiPSs suna komawa zuwa gurɓataccen lantarki ta hanyar electrolyte, yayin da electrons ke komawa zuwa gurɓataccen lantarki ta hanyar da'irar waje don samar da ƙarfe na lithium tare da ions lithium, kuma LiPSs an rage su zuwa sulfur a tabbataccen lantarki don kammala tsarin caji.
Tsarin fitarwa na batir lithium-sulfur galibi mataki ne, multi-electron, multi-phase complex electrochemical reaction on sulfur cathode, da LiPSs masu tsayin sarkar daban-daban suna canzawa zuwa juna yayin aikin cajin.A yayin aiwatar da fitarwa, ana nuna martanin da zai iya faruwa a madaidaicin lantarki a hoto na 2, kuma ana nuna martanin da aka yi a gurɓataccen lantarki a hoto na 3.
Fa'idodin batirin lithium-sulfur a bayyane yake, kamar ƙarfin ka'ida mai girma;babu iskar oxygen a cikin kayan, kuma yanayin juyin halittar oxygen ba zai faru ba, don haka aikin aminci yana da kyau;albarkatun sulfur suna da yawa kuma sulfur na asali yana da arha;yana da alaƙa da muhalli kuma yana da ƙarancin guba.Koyaya, batirin lithium-sulfur suma suna da wasu matsalolin ƙalubale, kamar tasirin lithium polysulfide shuttle;da rufi na elemental sulfur da fitar da kayayyakin;matsalar babban girma canje-canje;SEI maras tabbas da matsalolin tsaro da lithium anodes ya haifar;al'amarin fitar da kai, da sauransu.
A matsayin sabon ƙarni na tsarin batir na biyu, batirin lithium-air da baturan lithium-sulfur suna da ƙimar ƙayyadaddun iya aiki na ka'ida, kuma sun ja hankalin masu bincike da kasuwar batirin ta biyu.A halin yanzu, waɗannan batura biyu har yanzu suna fuskantar matsalolin kimiyya da fasaha da yawa.Suna cikin matakin farko na bincike na haɓaka baturi.Baya ga ƙayyadaddun iyawa da kwanciyar hankali na kayan cathode baturi da ke buƙatar haɓakawa, mahimman batutuwa kamar amincin baturi kuma suna buƙatar a warware su cikin gaggawa.A nan gaba, waɗannan sabbin nau'ikan batura guda biyu har yanzu suna buƙatar ci gaba da haɓaka fasaha don kawar da lahaninsu don buɗe fa'idodin aikace-aikacen.
Lokacin aikawa: Afrilu-07-2023