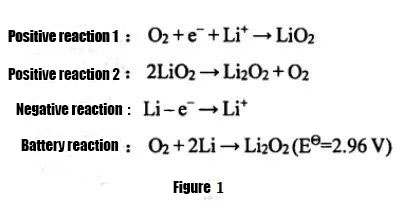01 What are lithium-air batteries and lithium-sulfur batteries?
① Li-air battery
The lithium-air battery uses oxygen as the positive electrode reactant and metal lithium as the negative electrode. It has a high theoretical energy density (3500wh/kg), and its actual energy density can reach 500-1000wh/kg, which is much higher than the conventional lithium-ion battery system. Lithium-air batteries are composed of positive electrodes, electrolytes and negative electrodes. In non-aqueous battery systems, pure oxygen is currently used as the reaction gas, so lithium-air batteries can also be called lithium-oxygen batteries.
In 1996, Abraham et al. successfully assembled the first non-aqueous lithium-air battery in the laboratory. Then researchers began to pay attention to the internal electrochemical reaction and mechanism of non-aqueous lithium-air batteries; in 2002, Read et al. found that the electrochemical performance of lithium-air batteries depended on the electrolyte solvent and air cathode materials; in 2006, Ogasawara et al. used Mass spectrometer, it was proved for the first time that Li2O2 was oxidized and oxygen was released during charging, which confirmed the electrochemical reversibility of Li2O2. Therefore, lithium-air batteries have received a lot of attention and rapid development.
② Lithium-sulfur battery
Lithium-sulfur battery is a secondary battery system based on the reversible reaction of high specific capacity sulfur (1675mAh/g) and lithium metal (3860mAh/g), with an average discharge voltage of about 2.15V. Its theoretical energy density can reach 2600wh/kg. Its raw materials have the advantages of low cost and environmental friendliness, so it has great development potential. The invention of lithium-sulfur batteries can be traced back to the 1960s, when Herbert and Ulam applied for a battery patent. The prototype of this lithium-sulfur battery used lithium or lithium alloy as the negative electrode material, sulfur as the positive electrode material and composed of aliphatic saturated amines. of electrolyte. A few years later, lithium-sulfur batteries were improved by introducing organic solvents such as PC, DMSO, and DMF, and 2.35-2.5V batteries were obtained. By the late 1980s, ethers were proven to be useful in lithium-sulfur batteries. In subsequent studies, the discovery of ether-based electrolytes, the use of LiNO3 as an electrolyte additive, and the proposal of carbon/sulfur composite positive electrodes have opened up the research boom of lithium-sulfur batteries.
02 Working principle of lithium-air battery and lithium-sulfur battery
① Li-air battery
According to the different states of the electrolyte used, lithium-air batteries can be divided into aqueous systems, organic systems, water-organic hybrid systems, and all-solid-state lithium-air batteries. Among them, due to the low specific capacity of lithium-air batteries using water-based electrolytes, difficulties in protecting lithium metal, and poor reversibility of the system, non-aqueous organic lithium-air batteries and all-solid-state lithium-air batteries are more widely used at present. Research. Non-aqueous lithium-air batteries were first proposed by Abraham and Z.Jiang in 1996. The discharge reaction equation is shown in Figure 1. The charging reaction is the opposite. The electrolyte mainly uses organic electrolyte or solid electrolyte, and the discharge product is mainly Li2O2 , the product is insoluble in the electrolyte, and is easy to accumulate on the air positive electrode, affecting the discharge capacity of the lithium-air battery.
Lithium-air batteries have the advantages of ultra-high energy density, environmental friendliness, and low price, but their research is still in its infancy, and there are still many problems to be solved, such as the catalysis of oxygen reduction reaction, the oxygen permeability and hydrophobicity of air electrodes, and the deactivation of air electrodes etc.
② Lithium-sulfur battery
Lithium-sulfur batteries mainly use elemental sulfur or sulfur-based compounds as the positive electrode material of the battery, and metallic lithium is mainly used for the negative electrode. During the discharge process, the metal lithium located at the negative electrode is oxidized to lose an electron and generate lithium ions; then the electrons are transferred to the positive electrode through the external circuit, and the generated lithium ions are also transferred to the positive electrode through the electrolyte to react with sulfur to form polysulfide. Lithium (LiPSs), and then further react to generate lithium sulfide to complete the discharge process. During the charging process, lithium ions in LiPSs return to the negative electrode through the electrolyte, while electrons return to the negative electrode through an external circuit to form lithium metal with lithium ions, and LiPSs are reduced to sulfur at the positive electrode to complete the charging process.
The discharge process of lithium-sulfur batteries is mainly a multi-step, multi-electron, multi-phase complex electrochemical reaction on the sulfur cathode, and LiPSs with different chain lengths are transformed into each other during the charge-discharge process. During the discharge process, the reaction that may occur at the positive electrode is shown in Figure 2, and the reaction at the negative electrode is shown in Figure 3.
The advantages of lithium-sulfur batteries are very obvious, such as very high theoretical capacity; there is no oxygen in the material, and oxygen evolution reaction will not occur, so the safety performance is good; sulfur resources are abundant and elemental sulfur is cheap; it is environmentally friendly and has low toxicity. However, lithium-sulfur batteries also have some challenging problems, such as the lithium polysulfide shuttle effect; the insulation of elemental sulfur and its discharge products; the problem of large volume changes; the unstable SEI and safety problems caused by lithium anodes; self-discharge phenomenon, etc.
As a new generation of secondary battery system, lithium-air batteries and lithium-sulfur batteries have very high theoretical specific capacity values, and have attracted extensive attention from researchers and the secondary battery market. At present, these two batteries are still facing many scientific and technical problems. They are in the early research stage of battery development. In addition to the specific capacity and stability of the battery cathode material needing to be further improved, key issues such as battery safety also need to be resolved urgently. In the future, these two new types of batteries still need continuous technical improvement to eliminate their defects in order to open up broader application prospects.
Post time: Apr-07-2023